Biology AS CIE
-
1-cell-structure10 主题
-
1-2-cells-as-the-basic-units-of-living-organisms AS viruses
-
1-2-cells-as-the-basic-units-of-living-organisms AS prokaryotic-v-eukaryotic-cells
-
1-2-cells-as-the-basic-units-of-living-organisms AS the-vital-role-of-atp
-
1-2-cells-as-the-basic-units-of-living-organisms AS animal-and-plant-cells
-
1-2-cells-as-the-basic-units-of-living-organisms AS eukaryotic-cell-structures-and-functions
-
1-1-the-microscope-in-cell-studies AS calculating-actual-size
-
1-1-the-microscope-in-cell-studies AS resolution-and-magnification
-
1-1-the-microscope-in-cell-studies AS eyepiece-graticules-and-stage-micrometers
-
1-1-the-microscope-in-cell-studies AS magnification-calculations
-
1-1-the-microscope-in-cell-studies AS the-microscope-in-cell-studies
-
1-2-cells-as-the-basic-units-of-living-organisms AS viruses
-
2-biological-molecules19 主题
-
2-4-water AS water-and-the-hydrogen-bond
-
2-4-water AS the-role-of-water-in-living-organisms
-
2-3-proteins AS collagen
-
2-3-proteins AS haemoglobin
-
2-3-proteins AS globular-and-fibrous-proteins
-
2-3-proteins AS protein-shape
-
2-3-proteins AS the-four-levels-of-protein-structure
-
2-3-proteins AS amino-acids-and-the-peptide-bond
-
2-2-carbohydrates-and-lipids AS phospholipids
-
2-2-carbohydrates-and-lipids AS triglycerides
-
2-2-carbohydrates-and-lipids AS cellulose
-
2-2-carbohydrates-and-lipids AS starch-and-glycogen
-
2-2-carbohydrates-and-lipids AS the-glycosidic-bond
-
2-2-carbohydrates-and-lipids AS reducing-and-non-reducing-sugars
-
2-2-carbohydrates-and-lipids AS covalent-bonds-in-polymers
-
2-2-carbohydrates-and-lipids AS biological-molecules-key-terms
-
2-1-testing-for-biological-molecules AS testing-for-non-reducing-sugars
-
2-1-testing-for-biological-molecules AS the-benedicts-test
-
2-1-testing-for-biological-molecules AS biological-molecule-tests
-
2-4-water AS water-and-the-hydrogen-bond
-
3-enzymes13 主题
-
3-2-factors-that-affect-enzyme-action AS enzyme-activity-immobilised-v-free
-
3-2-factors-that-affect-enzyme-action AS enzyme-inhibitors
-
3-2-factors-that-affect-enzyme-action AS vmax-and-the-michaelis-menten-constant
-
3-2-factors-that-affect-enzyme-action AS rate-inhibitor-concentration
-
3-2-factors-that-affect-enzyme-action AS rate-substrate-concentration
-
3-2-factors-that-affect-enzyme-action AS rate-enzyme-concentration
-
3-2-factors-that-affect-enzyme-action AS rate-ph
-
3-2-factors-that-affect-enzyme-action AS rate-temperature
-
3-1-mode-of-action-of-enzymes AS colorimetry
-
3-1-mode-of-action-of-enzymes AS measuring-enzyme-activity
-
3-1-mode-of-action-of-enzymes AS how-enzymes-work
-
3-1-mode-of-action-of-enzymes AS enzyme-action
-
3-1-mode-of-action-of-enzymes AS enzymes
-
3-2-factors-that-affect-enzyme-action AS enzyme-activity-immobilised-v-free
-
4-cell-membranes-and-transport16 主题
-
4-2-movement-into-and-out-of-cells AS comparing-osmosis-in-plants-and-animals
-
4-2-movement-into-and-out-of-cells AS osmosis-in-animals
-
4-2-movement-into-and-out-of-cells AS osmosis-in-plant-cells
-
4-2-movement-into-and-out-of-cells AS estimating-water-potential-in-plants
-
4-2-movement-into-and-out-of-cells AS investigating-surface-area
-
4-2-movement-into-and-out-of-cells AS surface-area-to-volume-ratios
-
4-2-movement-into-and-out-of-cells AS investigating-diffusion
-
4-2-movement-into-and-out-of-cells AS investigating-transport-processes-in-plants
-
4-2-movement-into-and-out-of-cells AS endocytosis-and-exocytosis
-
4-2-movement-into-and-out-of-cells AS active-transport
-
4-2-movement-into-and-out-of-cells AS osmosis
-
4-2-movement-into-and-out-of-cells AS diffusion
-
4-1-fluid-mosaic-membranes AS cell-signalling
-
4-1-fluid-mosaic-membranes AS the-cell-surface-membrane
-
4-1-fluid-mosaic-membranes AS components-of-cell-surface-membranes
-
4-1-fluid-mosaic-membranes AS the-fluid-mosaic-model
-
4-2-movement-into-and-out-of-cells AS comparing-osmosis-in-plants-and-animals
-
5-the-mitotic-cell-cycle8 主题
-
5-2-chromosome-behaviour-in-mitosis AS observing-mitosis
-
5-2-chromosome-behaviour-in-mitosis AS the-stages-of-mitosis
-
5-1-replication-and-division-of-nuclei-and-cells AS how-tumours-form
-
5-1-replication-and-division-of-nuclei-and-cells AS the-role-of-stem-cells
-
5-1-replication-and-division-of-nuclei-and-cells AS the-role-of-telomeres-
-
5-1-replication-and-division-of-nuclei-and-cells AS the-cell-cycle
-
5-1-replication-and-division-of-nuclei-and-cells AS mitosis
-
5-1-replication-and-division-of-nuclei-and-cells AS chromosome-structure
-
5-2-chromosome-behaviour-in-mitosis AS observing-mitosis
-
6-nucleic-acids-and-protein-synthesis9 主题
-
6-2-protein-synthesis AS gene-mutations
-
6-2-protein-synthesis AS transcription
-
6-2-protein-synthesis AS constructing-polypeptides
-
6-2-protein-synthesis AS the-universal-genetic-code
-
6-2-protein-synthesis AS from-gene-to-polypeptide
-
6-1-structure-of-nucleic-acids-and-replication-of-dna AS the-structure-of-rna
-
6-1-structure-of-nucleic-acids-and-replication-of-dna AS semi-conservative-dna-replication
-
6-1-structure-of-nucleic-acids-and-replication-of-dna AS the-structure-of-dna
-
6-1-structure-of-nucleic-acids-and-replication-of-dna AS nucleotides
-
6-2-protein-synthesis AS gene-mutations
-
7-transport-in-plants11 主题
-
7-2-transport-mechanisms AS phloem-mass-flow
-
7-2-transport-mechanisms AS the-sucrose-loading-mechanism
-
7-2-transport-mechanisms AS movement-in-the-phloem
-
7-2-transport-mechanisms AS xerophytic-plant-leaf-adaptations
-
7-2-transport-mechanisms AS water-and-the-transpiration-pull
-
7-2-transport-mechanisms AS transpiration-in-plants
-
7-2-transport-mechanisms AS water-and-mineral-ion-transport-in-plants
-
7-1-structure-of-transport-tissues AS phloem-sieve-tube-elements
-
7-1-structure-of-transport-tissues AS xylem-vessels-elements
-
7-1-structure-of-transport-tissues AS xylem-and-phloem-distribution
-
7-1-structure-of-transport-tissues AS plant-transverse-sections
-
7-2-transport-mechanisms AS phloem-mass-flow
-
8-transport-in-mammals16 主题
-
8-3-the-heart AS heart-action
-
8-3-the-heart AS the-cardiac-cycle
-
8-3-the-heart AS the-walls-of-the-heart
-
8-3-the-heart AS structure-of-the-heart
-
8-2-transport-of-oxygen-and-carbon-dioxide AS the-bohr-shift
-
8-2-transport-of-oxygen-and-carbon-dioxide AS the-oxygen-dissociation-curve
-
8-2-transport-of-oxygen-and-carbon-dioxide AS plasma-and-carbon-dioxide
-
8-2-transport-of-oxygen-and-carbon-dioxide AS the-chloride-shift
-
8-2-transport-of-oxygen-and-carbon-dioxide AS red-blood-cells-haemoglobin-and-oxygen
-
8-1-the-circulatory-system AS blood-tissue-fluid-and-lymph
-
8-1-the-circulatory-system AS the-role-of-water-in-circulation
-
8-1-the-circulatory-system AS cells-of-the-blood
-
8-1-the-circulatory-system AS blood-vessels-structures-and-functions
-
8-1-the-circulatory-system AS observing-and-drawing-blood-vessels
-
8-1-the-circulatory-system AS the-main-blood-vessels
-
8-1-the-circulatory-system AS circulatory-systems
-
8-3-the-heart AS heart-action
-
9-gas-exchange6 主题
-
9-1-the-gas-exchange-system AS gas-exchange-processes
-
9-1-the-gas-exchange-system AS structures-and-functions-of-the-gas-exchange-system
-
9-1-the-gas-exchange-system AS recognising-structures
-
9-1-the-gas-exchange-system AS recognising-tissues
-
9-1-the-gas-exchange-system AS distribution-of-tissues
-
9-1-the-gas-exchange-system AS the-human-gas-exchange-system
-
9-1-the-gas-exchange-system AS gas-exchange-processes
-
10-infectious-diseases6 主题
-
11-immunity10 主题
-
11-2-antibodies-and-vaccination AS vaccination-to-control-disease
-
11-2-antibodies-and-vaccination AS how-vaccines-work
-
11-2-antibodies-and-vaccination AS types-of-immunity
-
11-2-antibodies-and-vaccination AS uses-of-monoclonal-antibodies
-
11-2-antibodies-and-vaccination AS making-monoclonal-antibodies
-
11-2-antibodies-and-vaccination AS antibodies
-
11-1-the-immune-system AS memory-cells-and-immunity
-
11-1-the-immune-system AS primary-immune-response
-
11-1-the-immune-system AS antigens
-
11-1-the-immune-system AS phagocytes
-
11-2-antibodies-and-vaccination AS vaccination-to-control-disease
3-2-factors-that-affect-enzyme-action AS rate-ph
Exam code:9700
Rate: pH
-
All enzymes have an optimum pH which is a pH at which they operate best
-
Enzymes are denatured at extremes of pH
-
Hydrogen and ionic bonds hold the tertiary structure of the protein (i.e. the enzyme) together
-
Below and above the optimum pH of an enzyme, solutions with an excess of H+ ions (acidic solutions) and OH– ions (alkaline solutions) can cause these bonds to break
-
This alters the shape of the active site, which means enzyme-substrate complexes form less easily
-
Eventually, enzyme-substrate complexes can no longer form at all
-
At this point, complete denaturation of the enzyme has occurred
-
-
Where an enzyme functions, can be an indicator of its optimal environment:
-
E.g. pepsin is found in the stomach, an acidic environment at pH 2 (due to the presence of hydrochloric acid in the stomach’s gastric juice)
-
Pepsin’s optimum pH, not surprisingly, is pH 2
-
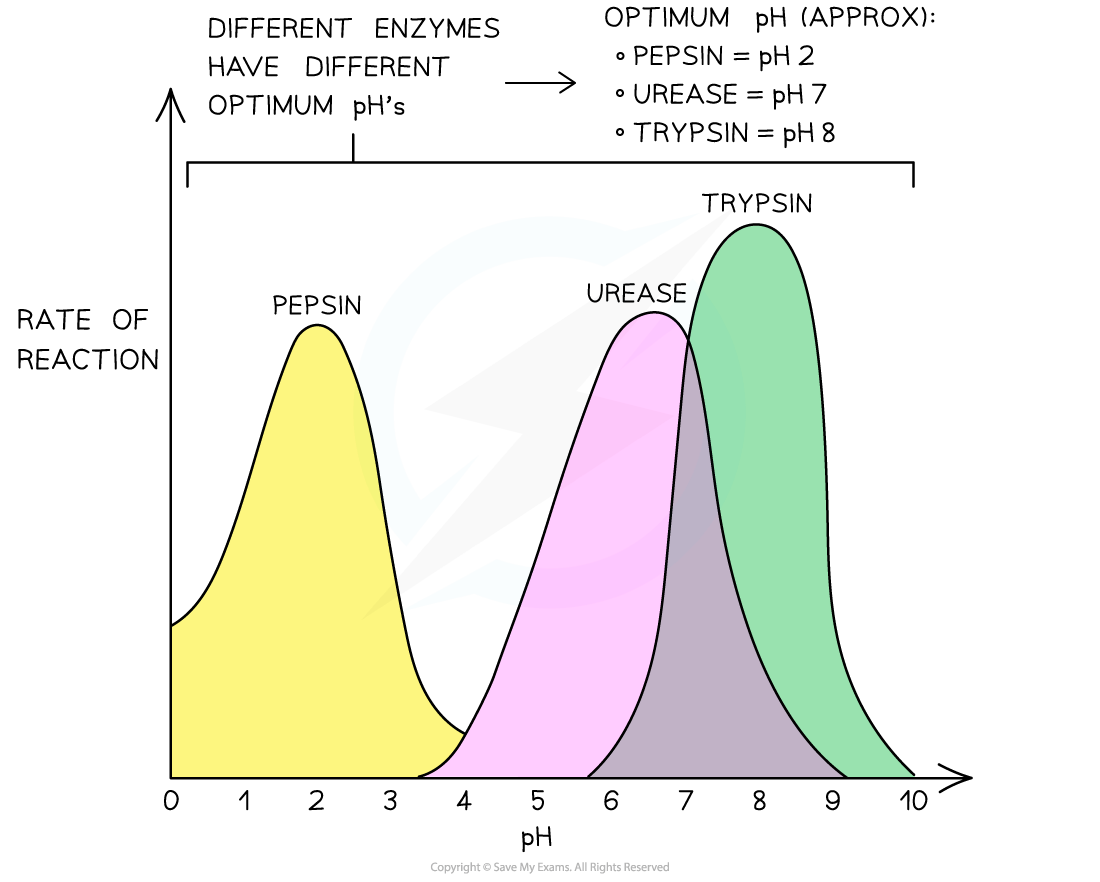
-
When investigating the effect of pH on the rate of an enzyme-catalysed reaction, you can use buffer solutions to measure the rate of reaction at different pH values:
-
Buffer solutions each have a specific pH
-
Buffer solutions maintain this specific pH, even if the reaction taking place would otherwise cause the pH of the reaction mixture to change
-
A measured volume of the buffer solution is added to the reaction mixture
-
This same volume (of each buffer solution being used) should be added for each pH value that is being investigated
-
Examiner Tips and Tricks
Temperature can both affect the speed at which molecules are moving (and therefore the number of enzyme-substrate collisions in a given time), and can denature enzymes (at high temperatures).
pH, however, does not affect collision rate but only disrupts the ability of the substrate to bind with the enzyme. This reduces the number of successful collisions until the active site changes shape so much that no more successful collisions can occur.

Responses