Biology AS CIE
-
1-cell-structure10 主题
-
1-2-cells-as-the-basic-units-of-living-organisms AS viruses
-
1-2-cells-as-the-basic-units-of-living-organisms AS prokaryotic-v-eukaryotic-cells
-
1-2-cells-as-the-basic-units-of-living-organisms AS the-vital-role-of-atp
-
1-2-cells-as-the-basic-units-of-living-organisms AS animal-and-plant-cells
-
1-2-cells-as-the-basic-units-of-living-organisms AS eukaryotic-cell-structures-and-functions
-
1-1-the-microscope-in-cell-studies AS calculating-actual-size
-
1-1-the-microscope-in-cell-studies AS resolution-and-magnification
-
1-1-the-microscope-in-cell-studies AS eyepiece-graticules-and-stage-micrometers
-
1-1-the-microscope-in-cell-studies AS magnification-calculations
-
1-1-the-microscope-in-cell-studies AS the-microscope-in-cell-studies
-
1-2-cells-as-the-basic-units-of-living-organisms AS viruses
-
2-biological-molecules19 主题
-
2-4-water AS water-and-the-hydrogen-bond
-
2-4-water AS the-role-of-water-in-living-organisms
-
2-3-proteins AS collagen
-
2-3-proteins AS haemoglobin
-
2-3-proteins AS globular-and-fibrous-proteins
-
2-3-proteins AS protein-shape
-
2-3-proteins AS the-four-levels-of-protein-structure
-
2-3-proteins AS amino-acids-and-the-peptide-bond
-
2-2-carbohydrates-and-lipids AS phospholipids
-
2-2-carbohydrates-and-lipids AS triglycerides
-
2-2-carbohydrates-and-lipids AS cellulose
-
2-2-carbohydrates-and-lipids AS starch-and-glycogen
-
2-2-carbohydrates-and-lipids AS the-glycosidic-bond
-
2-2-carbohydrates-and-lipids AS reducing-and-non-reducing-sugars
-
2-2-carbohydrates-and-lipids AS covalent-bonds-in-polymers
-
2-2-carbohydrates-and-lipids AS biological-molecules-key-terms
-
2-1-testing-for-biological-molecules AS testing-for-non-reducing-sugars
-
2-1-testing-for-biological-molecules AS the-benedicts-test
-
2-1-testing-for-biological-molecules AS biological-molecule-tests
-
2-4-water AS water-and-the-hydrogen-bond
-
3-enzymes13 主题
-
3-2-factors-that-affect-enzyme-action AS enzyme-activity-immobilised-v-free
-
3-2-factors-that-affect-enzyme-action AS enzyme-inhibitors
-
3-2-factors-that-affect-enzyme-action AS vmax-and-the-michaelis-menten-constant
-
3-2-factors-that-affect-enzyme-action AS rate-inhibitor-concentration
-
3-2-factors-that-affect-enzyme-action AS rate-substrate-concentration
-
3-2-factors-that-affect-enzyme-action AS rate-enzyme-concentration
-
3-2-factors-that-affect-enzyme-action AS rate-ph
-
3-2-factors-that-affect-enzyme-action AS rate-temperature
-
3-1-mode-of-action-of-enzymes AS colorimetry
-
3-1-mode-of-action-of-enzymes AS measuring-enzyme-activity
-
3-1-mode-of-action-of-enzymes AS how-enzymes-work
-
3-1-mode-of-action-of-enzymes AS enzyme-action
-
3-1-mode-of-action-of-enzymes AS enzymes
-
3-2-factors-that-affect-enzyme-action AS enzyme-activity-immobilised-v-free
-
4-cell-membranes-and-transport16 主题
-
4-2-movement-into-and-out-of-cells AS comparing-osmosis-in-plants-and-animals
-
4-2-movement-into-and-out-of-cells AS osmosis-in-animals
-
4-2-movement-into-and-out-of-cells AS osmosis-in-plant-cells
-
4-2-movement-into-and-out-of-cells AS estimating-water-potential-in-plants
-
4-2-movement-into-and-out-of-cells AS investigating-surface-area
-
4-2-movement-into-and-out-of-cells AS surface-area-to-volume-ratios
-
4-2-movement-into-and-out-of-cells AS investigating-diffusion
-
4-2-movement-into-and-out-of-cells AS investigating-transport-processes-in-plants
-
4-2-movement-into-and-out-of-cells AS endocytosis-and-exocytosis
-
4-2-movement-into-and-out-of-cells AS active-transport
-
4-2-movement-into-and-out-of-cells AS osmosis
-
4-2-movement-into-and-out-of-cells AS diffusion
-
4-1-fluid-mosaic-membranes AS cell-signalling
-
4-1-fluid-mosaic-membranes AS the-cell-surface-membrane
-
4-1-fluid-mosaic-membranes AS components-of-cell-surface-membranes
-
4-1-fluid-mosaic-membranes AS the-fluid-mosaic-model
-
4-2-movement-into-and-out-of-cells AS comparing-osmosis-in-plants-and-animals
-
5-the-mitotic-cell-cycle8 主题
-
5-2-chromosome-behaviour-in-mitosis AS observing-mitosis
-
5-2-chromosome-behaviour-in-mitosis AS the-stages-of-mitosis
-
5-1-replication-and-division-of-nuclei-and-cells AS how-tumours-form
-
5-1-replication-and-division-of-nuclei-and-cells AS the-role-of-stem-cells
-
5-1-replication-and-division-of-nuclei-and-cells AS the-role-of-telomeres-
-
5-1-replication-and-division-of-nuclei-and-cells AS the-cell-cycle
-
5-1-replication-and-division-of-nuclei-and-cells AS mitosis
-
5-1-replication-and-division-of-nuclei-and-cells AS chromosome-structure
-
5-2-chromosome-behaviour-in-mitosis AS observing-mitosis
-
6-nucleic-acids-and-protein-synthesis9 主题
-
6-2-protein-synthesis AS gene-mutations
-
6-2-protein-synthesis AS transcription
-
6-2-protein-synthesis AS constructing-polypeptides
-
6-2-protein-synthesis AS the-universal-genetic-code
-
6-2-protein-synthesis AS from-gene-to-polypeptide
-
6-1-structure-of-nucleic-acids-and-replication-of-dna AS the-structure-of-rna
-
6-1-structure-of-nucleic-acids-and-replication-of-dna AS semi-conservative-dna-replication
-
6-1-structure-of-nucleic-acids-and-replication-of-dna AS the-structure-of-dna
-
6-1-structure-of-nucleic-acids-and-replication-of-dna AS nucleotides
-
6-2-protein-synthesis AS gene-mutations
-
7-transport-in-plants11 主题
-
7-2-transport-mechanisms AS phloem-mass-flow
-
7-2-transport-mechanisms AS the-sucrose-loading-mechanism
-
7-2-transport-mechanisms AS movement-in-the-phloem
-
7-2-transport-mechanisms AS xerophytic-plant-leaf-adaptations
-
7-2-transport-mechanisms AS water-and-the-transpiration-pull
-
7-2-transport-mechanisms AS transpiration-in-plants
-
7-2-transport-mechanisms AS water-and-mineral-ion-transport-in-plants
-
7-1-structure-of-transport-tissues AS phloem-sieve-tube-elements
-
7-1-structure-of-transport-tissues AS xylem-vessels-elements
-
7-1-structure-of-transport-tissues AS xylem-and-phloem-distribution
-
7-1-structure-of-transport-tissues AS plant-transverse-sections
-
7-2-transport-mechanisms AS phloem-mass-flow
-
8-transport-in-mammals16 主题
-
8-3-the-heart AS heart-action
-
8-3-the-heart AS the-cardiac-cycle
-
8-3-the-heart AS the-walls-of-the-heart
-
8-3-the-heart AS structure-of-the-heart
-
8-2-transport-of-oxygen-and-carbon-dioxide AS the-bohr-shift
-
8-2-transport-of-oxygen-and-carbon-dioxide AS the-oxygen-dissociation-curve
-
8-2-transport-of-oxygen-and-carbon-dioxide AS plasma-and-carbon-dioxide
-
8-2-transport-of-oxygen-and-carbon-dioxide AS the-chloride-shift
-
8-2-transport-of-oxygen-and-carbon-dioxide AS red-blood-cells-haemoglobin-and-oxygen
-
8-1-the-circulatory-system AS blood-tissue-fluid-and-lymph
-
8-1-the-circulatory-system AS the-role-of-water-in-circulation
-
8-1-the-circulatory-system AS cells-of-the-blood
-
8-1-the-circulatory-system AS blood-vessels-structures-and-functions
-
8-1-the-circulatory-system AS observing-and-drawing-blood-vessels
-
8-1-the-circulatory-system AS the-main-blood-vessels
-
8-1-the-circulatory-system AS circulatory-systems
-
8-3-the-heart AS heart-action
-
9-gas-exchange6 主题
-
9-1-the-gas-exchange-system AS gas-exchange-processes
-
9-1-the-gas-exchange-system AS structures-and-functions-of-the-gas-exchange-system
-
9-1-the-gas-exchange-system AS recognising-structures
-
9-1-the-gas-exchange-system AS recognising-tissues
-
9-1-the-gas-exchange-system AS distribution-of-tissues
-
9-1-the-gas-exchange-system AS the-human-gas-exchange-system
-
9-1-the-gas-exchange-system AS gas-exchange-processes
-
10-infectious-diseases6 主题
-
11-immunity10 主题
-
11-2-antibodies-and-vaccination AS vaccination-to-control-disease
-
11-2-antibodies-and-vaccination AS how-vaccines-work
-
11-2-antibodies-and-vaccination AS types-of-immunity
-
11-2-antibodies-and-vaccination AS uses-of-monoclonal-antibodies
-
11-2-antibodies-and-vaccination AS making-monoclonal-antibodies
-
11-2-antibodies-and-vaccination AS antibodies
-
11-1-the-immune-system AS memory-cells-and-immunity
-
11-1-the-immune-system AS primary-immune-response
-
11-1-the-immune-system AS antigens
-
11-1-the-immune-system AS phagocytes
-
11-2-antibodies-and-vaccination AS vaccination-to-control-disease
2-3-proteins AS protein-shape
Exam code:9700
Proteins: interactions & shape
-
A polypeptide chain will fold differently due to the interactions (and the bonds that form) between R groups
-
The three-dimensional configuration that forms is called the tertiary structure of a protein
-
-
Each of the twenty amino acids that make up proteins has a unique R group
-
This means that many different interactions can occur creating a vast range of protein configurations and therefore functions
-
-
Within proteins with a tertiary structure the following bonds occur:
-
Strong covalent disulfide
-
Ionic
-
Weak hydrogen
-
Weak hydrophobic interactions
-
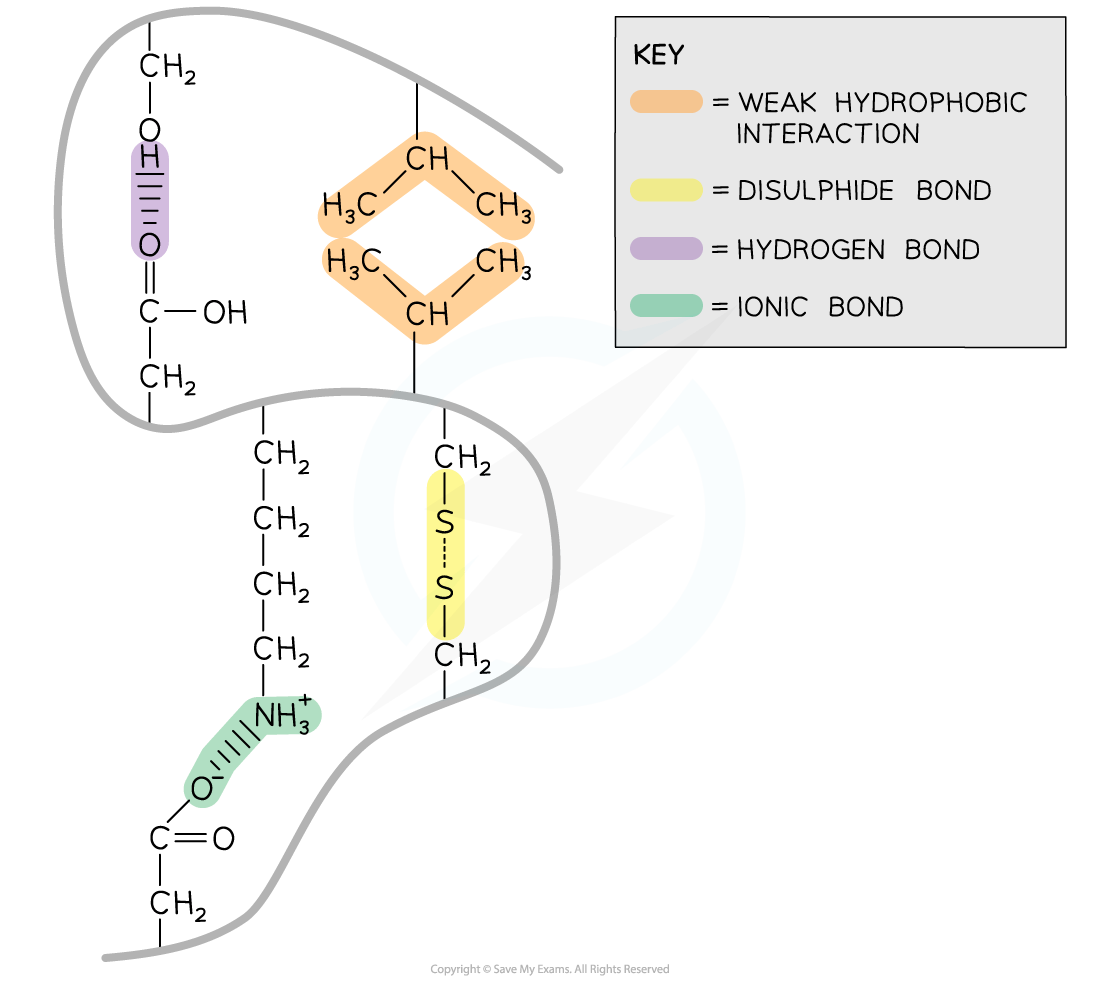
Disulfide
-
Disulfide bonds are strong covalent bonds that form between two cysteine R groups (as this is the only amino acid with an available sulfur atom in its R group)
-
These bonds are the strongest within a protein, but occur less frequently, and help stabilise the proteins
-
These are also known as disulfide bridges
-
Can be broken by reduction
-
Disulfide bonds are common in proteins secreted from cells e.g. insulin
Ionic
-
Ionic bonds form between positively charged (amine group -NH3+) and negatively charged (carboxylic acid -COO–) R groups
-
Ionic bonds are stronger than hydrogen bonds but they are not common
-
These bonds are broken by pH changes
Hydrogen
-
Hydrogen bonds form between strongly polar R groups. These are the weakest bonds that form but the most common as they form between a wide variety of R groups
Hydrophobic interactions
-
Hydrophobic interactions form between the non-polar (hydrophobic) R groups within the interior of proteins
Examiner Tips and Tricks
Note that an interaction is not the same as a bond. An interaction refers to a low energy attraction between two groups, whereas a bond is high energy and typically involves the electrons of the molecules concerned.
You need to be able to determine which bonds are found in tertiary structures and recognise them in diagrams.

Responses